As of 2024, 19 bispecific antibody drugs have been approved globally, with over 200 candidates in clinical development and an annual growth rate exceeding 30%. Current focus remains on oncology, while expanding into hematologic diseases, autoimmune disorders and other areas. Leveraging diverse structural formats (e.g., IgG-like, BiTE®), BsAbs enable synergistic targeting and immune cell bridging, demonstrating superior efficacy versus conventional therapies. Emerging technologies like BsAb-ADCs are driving applications in neurodegenerative diseases and beyond.
Sanyou Bio offers a validated collection of 73 reference BsAb covering major platforms (CrossMab, BiTE®, etc.), supporting end-to-end R&D from candidate screening to preclinical evaluation. SY-CRS-BsAb solutions accelerate development by addressing key technical challenges and improving efficiency.
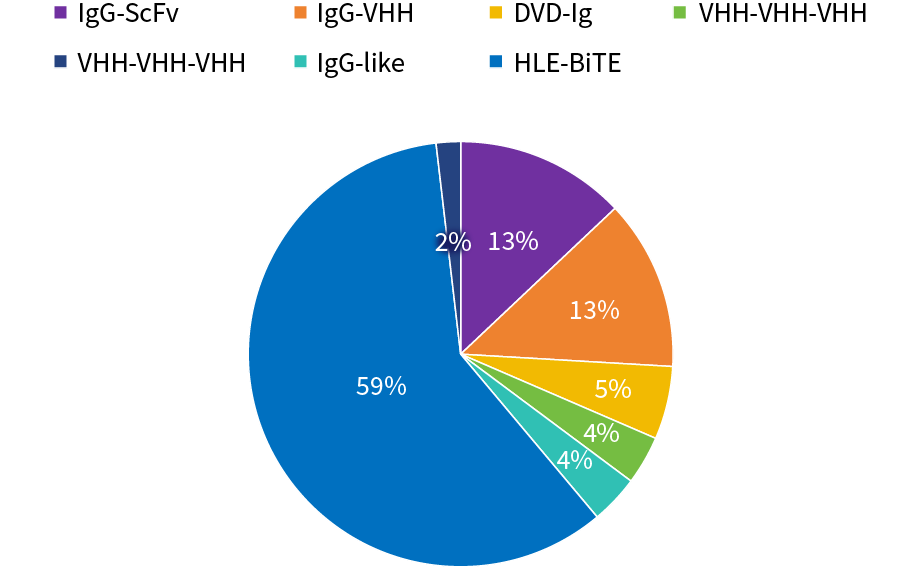
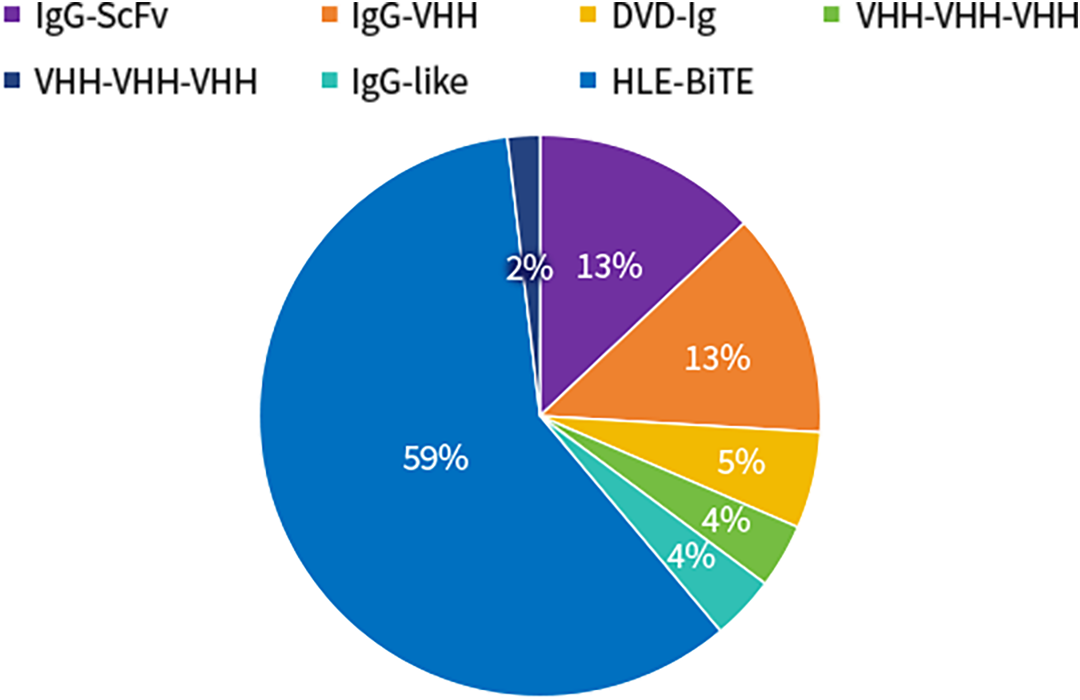
SY-CRS-BsAb Isotype Distribution
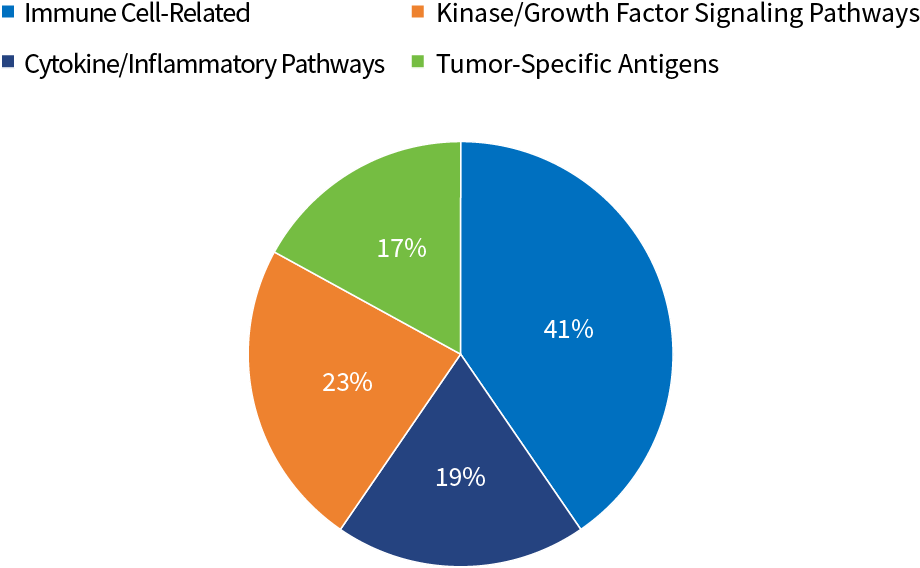
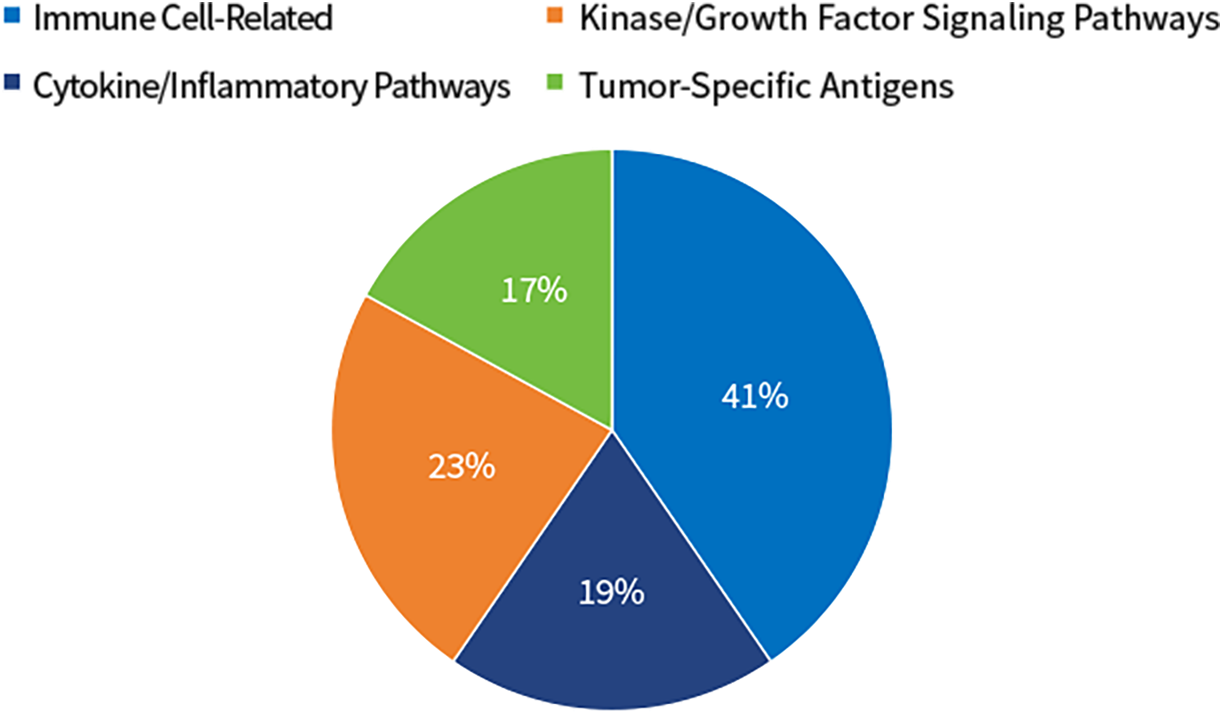
SY-CRS-BsAb Target Distribution
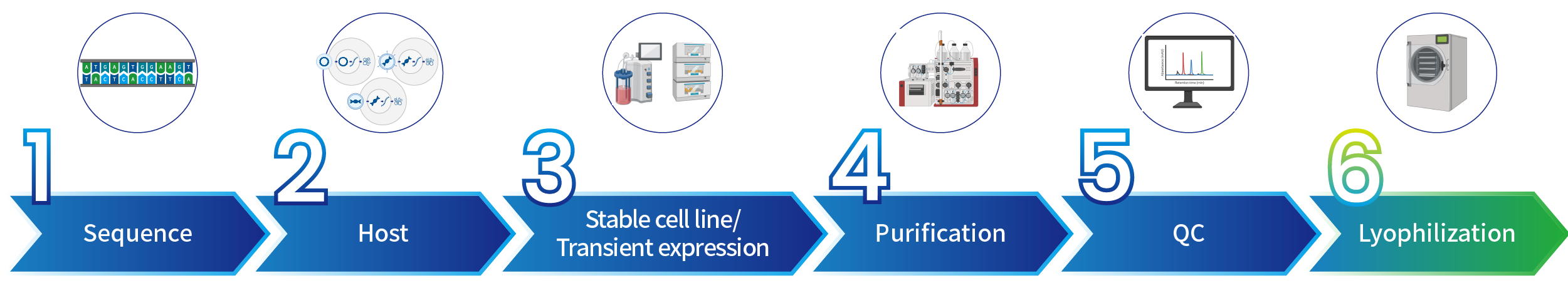
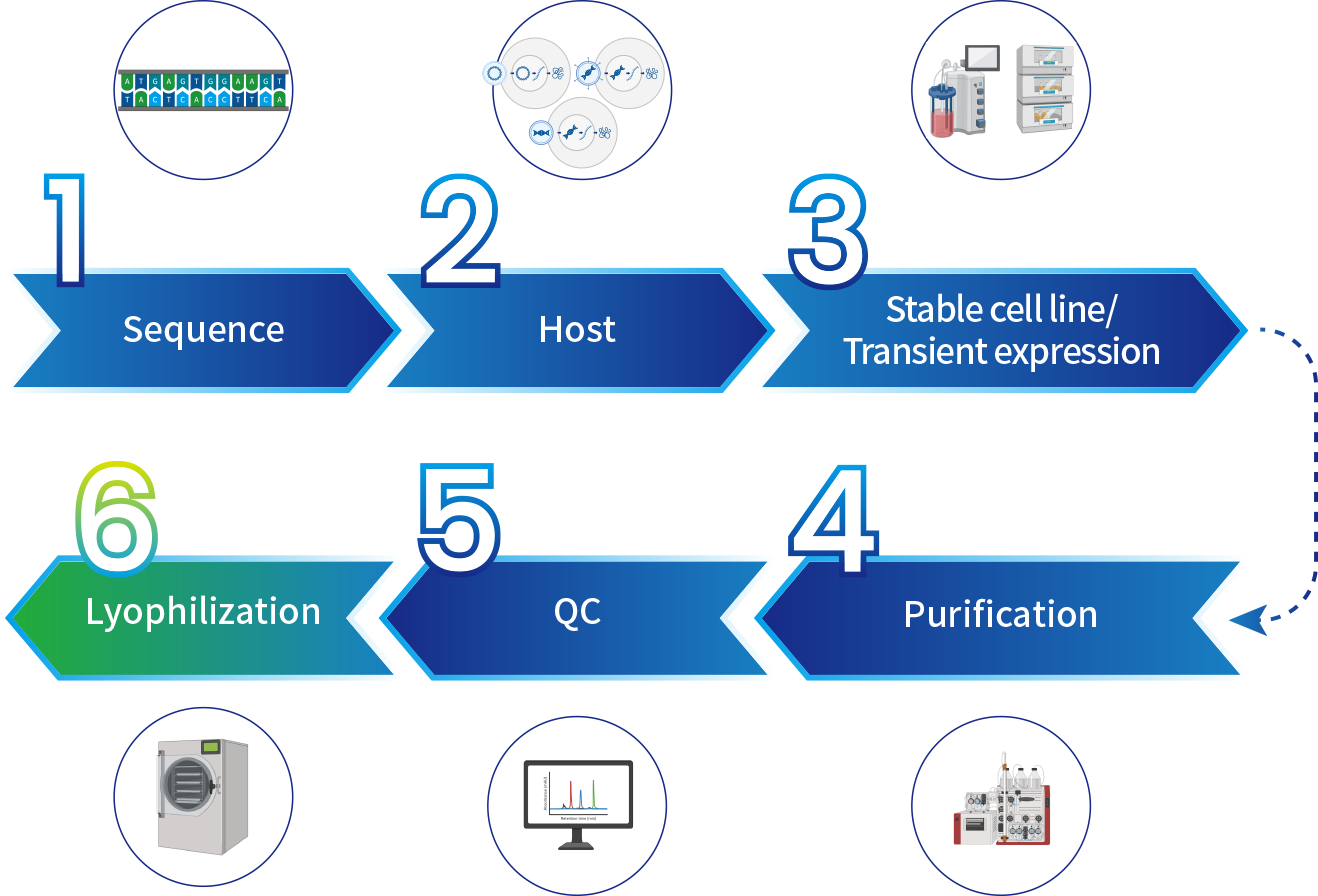
Manufacturing Process
Quality Management System
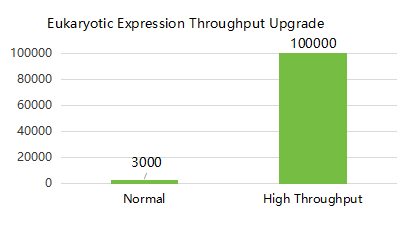
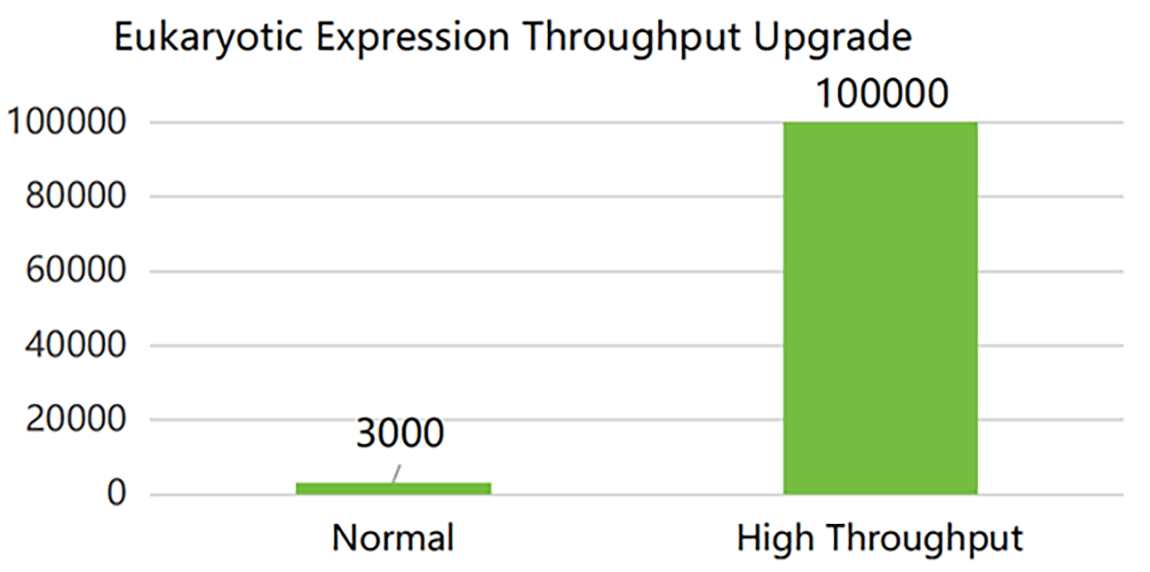
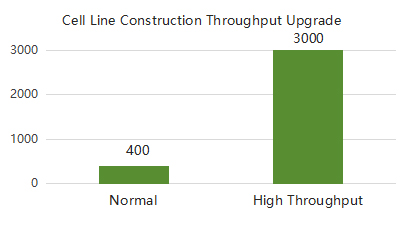
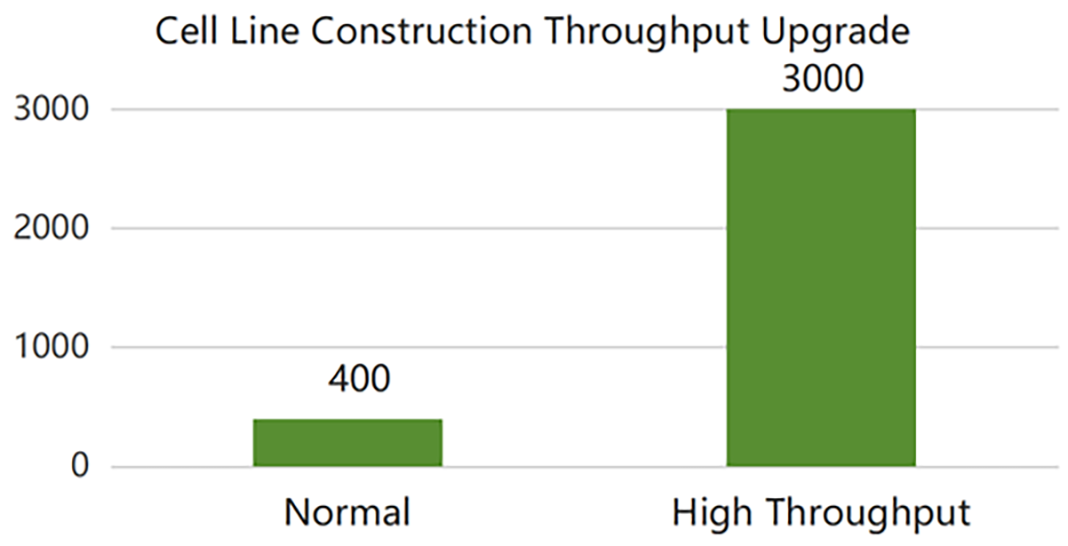
SanYou Bio’s advanced antibody production system features high-end international equipment and full capabilities in plasmid construction, protein expression, and purification. Operating under GMP-grade host cells and Grade C+A cleanroom conditions, it follows 400+ standardized SOPs to ensure high-throughput, efficient delivery. All processes comply with ISO9001 and ISO27001, with strict quality control to guarantee antibody quality.
Data | SY-CRS-BsAb |
|---|
Concentration | √ |
Purity: SDS-PAGE | √ |
Purity: SEC-HPLC | √ |
LC-MS | √ |
Endotoxin | √ |
ELISA Binding | √ |
FACS Binding | √ |
Funtional Assay | √ |
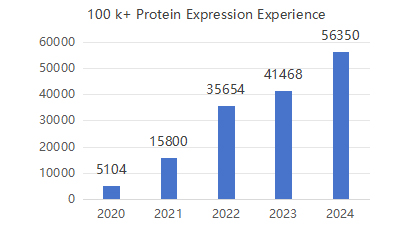
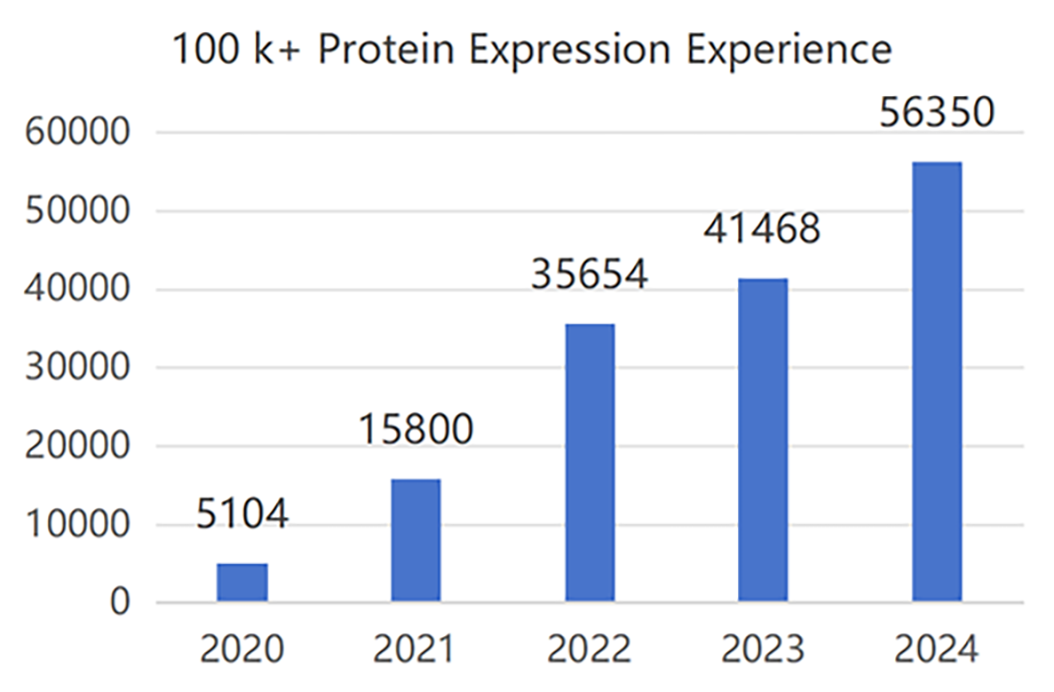
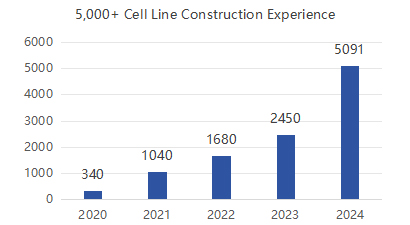
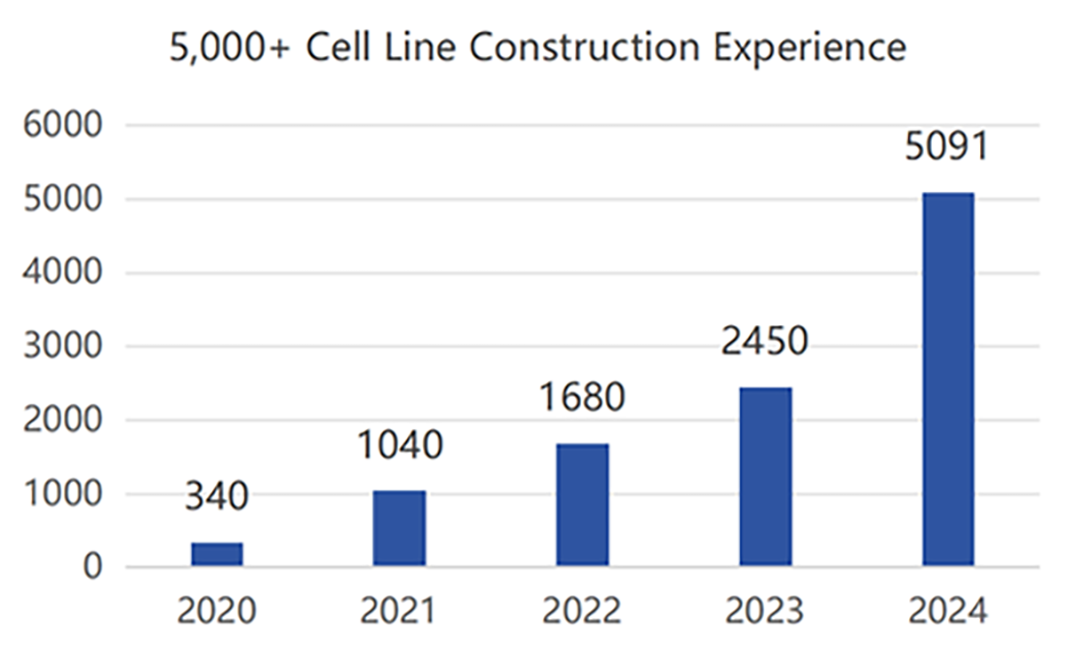
Production Experience

 Overview
Overview Configuration
Configuration Product
Product Database
Database Brochure
Brochure Promotion
Promotion